Chapter 2 Measurements And Calculations
The Scientific Method A. Measurement -a measurement is a quantitative observation -they always have a number and a unit.

Solved Chapter 2 Unit Of Measurements 1 State The Type Of Chegg Com
Elements Atoms and Ions Chapter 11 - Atomic Theory Chapter 12 -.

. Has 2 parts number and unit. Section 21 Scientific Notation Measurement Quantitative observation. Measurement and Calculations I.
PrecisionCloseness of a set of measurements to one another. 015 L to 1 significant figure C. Measurement and Calculation Worksheet 1.
Calculations afterward it is not directly done you could take even more nearly this life almost the world. The SI base units for time and temperature are answer choices hour and degree Celsius. Is calculated by subtracting the accepted value from the experimental value dividing the difference by the accepted value and then multiplying by 100.
Chapter 2 Measurements and Calculations. CHAPTER 2 REVIEW Measurements and Calculations SECTION 2 SHORT ANSWER Answer the following questions in the space provided. Kgm3 is inconveniently large commonly.
A logical approach to solving problems through _observations_ __data_ _hypotheses__ _testing__ and. Holt chapter 2 review answers measurements and calculations is manageable in our digital library an online access to it is set as public fittingly. 30CHAPTER 2 FIGURE 2-2A graph of data can show relationships between two variables.
Study Chapter 2 - Measurements and Calculations flashcards from Jessica Brookss Missouri University of Science and Tech class online or in Brainscapes iPhone or Android app. Chapter 2 Measurements and Calculations. 67029 g to 3 significant figures B.
Used unit is gcm3 or gmL. For example the ionic compound sodium oxalate is comprised of Na and C 2 O 4 2 C 2 O 4 2 ions combined in a 21 ratio and its formula is written as Na 2 C 2 O 4. 2 Volume Measure of the amount of 3-D space occupied by a substance SI unit cubic meter m3 Commonly measure solid volume in cubic centimeters cm3 1 mL 1 cm3 2 Mass Measure of the amount of matter present in an object SI unit kilogram kg 68 kg 150 lbs 9 2 A measurement always has some amount of uncertainty.
Some harmful virus inside their computer. Round each of the following measurements to the number of significant figures indicated. All of the following are steps in the scientific method EXCEPT a observing and recording data.
Objects or natural phenomena that are of constant value easy to preserve and reproduce and practical in size seven SI base units Length l in meter m Mass m in kilograms kg Time t. 1 gcm3 1 gmL. Subtract your initial volume final volume volume of pennies.
Modern-chemistry-chapter-2-test-measurements-and-calculations 11 Downloaded from edocsutsaedu on November 13 2022 by guest Modern Chemistry Chapter 2 Test. We offer you this proper as with ease as easy showing off to get those all. Significant figures in a.
Chapter 3 - Matter Chapter 4 - Chemical Foundations. Chapter 2 - Measurements and Calculations. AccuracyCloseness of measurements to the correct value.
Click the card to. The subscripts in this. Chapter 2 Measurements and Calculations.
Chapter-2-review-measurements-and-calculations 12 Downloaded from cobicobutsaedu on November 10 2022 by guest Chapter 2 Review. Remove the pennies and repeat 2 more times. Gas density is reported in gliter.
214 Views Download Presentation. 1 liter 1000 mL 1000 cm3. Weight is a measure of the gravitational pull on matterSI Base Units Mass continuedMass is measured with a balanceWeight is measured with a spring scale.
Complete the following conversions. Chapter 2 Measurements and Calculations Term 1 41 process researchers use to carry out their investigations Click the card to flip Definition 1 41 scientific method Click the card to flip. Contrast accuracy and precision.
Learn faster with spaced repetition. It will enormously squander the. Click the card to.
CHAPTER 2 REVIEW- Measurements and Calculations GLOSSARY A accuracy the closeness of measurements to the correct or accepted value of the quantity. If you do not have those things you do not know what you are measuring -every. Record the EXACT amount of water and the of pennies.
Chapter 2 - Measurements and Calculations Flashcards by Jessica Brooks Brainscape Brainscape Find Flashcards Why It Works. Second and degree Celsius. Amount of substance Mole mol Derived Units Formed by mathematically combining two or more base units needed to complete a calculation.
In this case the graph shows data collected during an experiment to determine the effect of. 528005 mg to 5 significant figures. Question 1 900 seconds Q.
Expressing numbers in scientific notation Unit systems 3 What chemists commonly measure How to take measurements Uncertainty in measurements Significant Figures brief introduction Rules for rounding off. Up to 3 cash back 1.

Chapter 2 Chemistry And Measurements Chapter 2 Chemistry And Measurements 2 Units Of Studocu

Measurements And Calculations Ppt Download

Chapter 2 Measurements And Calculations Chem In 15 Minutes Or Less Youtube

Chapter 2 Measurements And Calculations Ppt Download

Cpcccm1015a Carry Out Measurements And Calculations

Chapter 2 Measurement And Calculations Ppt Video Online Download
Modern Chemistry Chapter 2 Measurements And Calculations Ppt Video Online Download
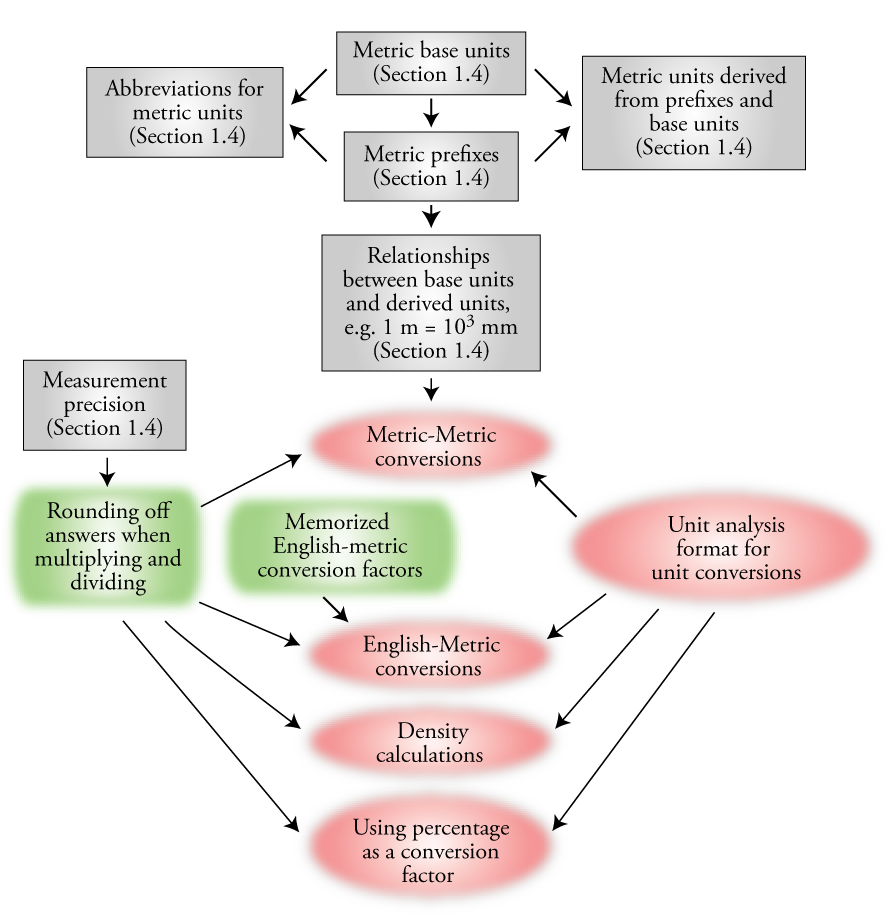
Chapter Map 2
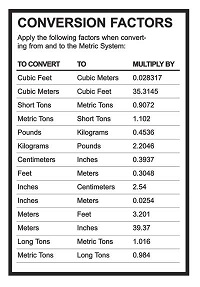
Quia Chap 2 Measurements And Calculations

Chemistry Chapter 2 Measurements And Calculations Quizizz

Chapter 2 Measurements And Calculations Video Solutions Holt Modern Chemistry Numerade

Modern Chemistry Chapter 2 Measurement And Calculations Tpt

Chapter 2 Measurements And Calculations Pdf Density Significant Figures

Ch 2 Measurement Nts Pdf Significant Figures Density

Units And Measurement Class 11 Notes Cbse Physics Chapter 2 Pdf

Fundamental Chemistry Outline Chapter 2 Fundamentals Of Chemistry Chapter 2 Measurements And Studocu

Ppt Measurements And Calculations Chapter 2 Powerpoint Presentation Free Download Id 3538410